 |
General Technology and ScienceApplying Natures PrinciplesAt the center of the evaporative cooling process and the Earth's climate moderating processes used by Mother Nature is water. From a weather and climate perspective, the existence of water in its three physical states (ice, liquid water, and water vapor), the behavior of water vapor as a perfect gas, and waters high characteristic values of thermal conductance, specific heat, and latent heat of vaporization are the reason that it is a major driver in determining and mediating the observed temperatures, barometric pressures, and wind currents throughout the globe. From an evaporative cooling perspective it is the evaporation of water that cools the outside air as it passes through the cooler media to provide conditioned air for improved equipment reliability and human comfort and productivity in hot climates. Understanding the Scientific Laws and PrinciplesThe objective of this web page is not to provide a comprehensive discussion of the scientific laws and engineering principals that govern the design, operation, and performance of evaporative coolers. Rather, the objective is to provide a focused overview with additional detail on the more pertinent laws and principles that drive cooler performance and are behind the significant improvements delivered by AZFlow coolers. To this end we present information on Psychrometry, Heat Transfer and Fluid Flow, Salt Scale Formation, and Corrosion. PsychrometryEvaporative cooler design and performance is driven by the thermodynamic behavior of moist air. Psychrometry which is the field of science dealing with the properties and thermodynamic behavior of moist air is therefore at the foundation of evaporative cooler design and performance. The following psychrometry principals and laws underlie the moist air properties and thermodynamic behaviors.
Moist Air PropertiesThe following are key characterization properties for moist air that are used in the design, evaluation, and operation of evaporative cooling and other cooling processes.
Fluid DynamicsFluid dynamic behavior of air and water in an evaporative cooler has a significant impact on the performance of evaporative coolers. Namely, having a uniform distribution of the proper proportions of air and water throughout the media to satisfy the thermodynamic requirements of the cooling process is critical to achieving the highest levels of performance. The following principles and laws govern the behavior and properties of fluids in motion and therefore the ability to distribute the water and air uniformly and in the desired proportions.
Hard Water and Scale FormationIn most of the US the water from rivers, lakes, and wells is classified as "Hard" to "Very Hard". Water hardness is a measure of the mineral content of water. The simplest way to determine if water is hard or soft is to use soap and attempt to create lather. Soft water lathers much more readily than hard water. Hard water typically contains high levels of metal ions in the form of dissolved calcium (Ca) and magnesium (Mg) carbonate salts. While these salts are usually the dominant constituents, several other metals may be present and bicarbonate and sulfate salt forms may be present. The common source of these minerals is Mother Earth as it undergoes weathering and erosion processes. In particular, the weathering / erosion of limestone CaCO3 and silicate CaSiO3 rich land mass. This activity is part of the Earth's Carbon Cycle with the atmosphere, biosphere, and oceans serving as mobile reservoirs and the rock mass acting as long term reservoir where most of the Carbon resides. From the perspective of designing and operating an evaporative cooler, the hardness of the water is important since it is a critical factor that drives the rate of scale production. This scale formation is an important concern since the existence of scale on evaporative cooler media results in reduced heat transfer and therefore non reversible reduction in cooler performance. The following factors govern scale formation during the evaporation of water and therefore play an important role in the evaporative cooling process.
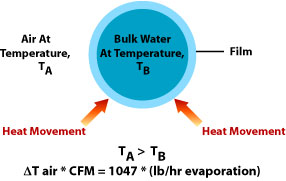
Energy and the Evaporative Cooling ProcessAs air that is below its saturation conditions (< 100% relative humidity) passes over a source of water, the water evaporates. When water evaporates it changes state from liquid to vapor; these two physical states of water exist at different internal energy levels while the sensible temperature and pressure remain the same; this difference in internal energy is called the latent heat of vaporization. The actual value for the latent heat of vaporization varies as the pressure varies (decreases with increasing pressure). A significant characteristic of water is that this latent heat of vaporization throughout its full range is the highest of any earthly substance (approximately 1050 BTU/lb at 75° F and sea level). As water evaporates, energy is drawn from the surrounding air and water. If there is no external source of energy to replace the energy that is used to evaporate the water, the energy of the surrounding air and water are reduced (the temperatures are reduced). The energy can only flow from a higher energy state to a lower energy state. Therefore, there is a limit to the level of cooling that can take place. The limit for direct evaporative cooling is that the dry bulb temperature leaving the cooler can not be less that the wet bulb temperature of the air entering the cooler. The temperature of the water that exits the cooler is also limited to this wet bulb temperature. Within this limit, cooling takes place as energy is drawn out of the air at a rate of one BTU for every pound of dry air that is cooled 4.7° F and one BTU for every pound of water that is cooled 1° F. As a rough example of the order of magnitude of the cooling effectiveness of this process we can assume a case where all energy comes from the surrounding air to cool it. If we further assume that we evaporate 1 pound of water (0.12 gallons) we would cool about 262 pounds (3550 cubic feet) of air 18.8° F. Psychrometric ChartsPsychrometric charts are one of the most widely used graphical devices of the refrigerating and air conditioning industry. Their principle purpose is to graphically portray the relation between the major properties of moist air: dry-bulb temperature, wet-bulb temperature, dew point temperature, humidity ratio, enthalpy, and specific volume. This graphical characterization provides a short cut process to predict and analyze the performance of HVAC cooling systems. In particular, armed with this chart one can locate the point that represents current or design cooler inlet conditions and predict the output conditions for various cooler operations. For example, heating or cooling without adding or removing moisture (the heat exchanger in an indirect cooling cycle) will follow the constant moisture or constant humidity ratio line, and direct cycle evaporative cooling follows the wet bulb temperature line. |
